Amphipathic Molecules and Phospholipid Bilayers:
Some larger molecules have regions that are hydrophilic (and will thus dissolve into water) and other regions that are hydrophobic (and thus won't). A good example is a
phospholipid molecule. Such a molecule is said to be
amphipathic.
A phospholipid molecule has a "head" that is strongly polar (hydrophilic) and so is attracted to water molecules. But it also has 2 nonpolar (hydrophobic) "tails" that are, in effect, repelled by water.
When placed into water, phospholipid molecules will therefore spontaneously organize into a
bilayer. The hydrophilic "heads" face outward, toward the water, and the hydrophobic "tails" face inward, away from the water.
You'll doubtless have noticed that this means the central region of a phospholipid bilayer is hydrophobic. Thus most polar and ionic substances cannot cross phospholipid bilayers, because they can't pass through the hydrophobic interior.
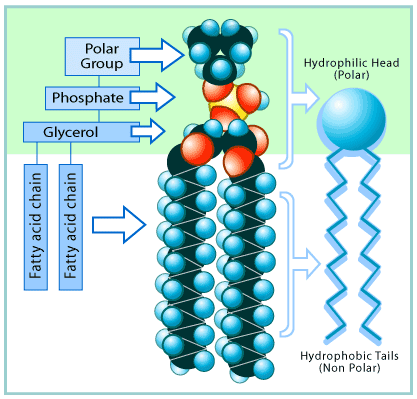
A phospholipid molecule. Note the polar (hydrophilic) "head" and the nonpolar (hydrophobic) "tails."

A Phospholipid Bilayer.
When placed into water, phospholipid molecules spontaneously organize into a bilayer, with the hydrophilic "heads"
facing outward, toward the surrounding water and the hydrophobic "tails" facing inward, away from the water.